Scope of the Nutrition and maternal health project
On this page
Skip the menu of subheadings on this page.This is a paper for discussion. It does not reflect the views of the Committee and should not be cited.
Introduction
1. The Scientific Advisory Committee on Nutrition (SACN) last considered the maternal diet and nutrition in relation to offspring health in its reports on ‘The influence of maternal, foetal and child nutrition on the development of chronic disease in later life’ (SACN, 2011) and on ‘Feeding in the first year of life’ (SACN, 2018). In the latter report, the impact of breastfeeding on maternal health was also considered. In 2019, SACN agreed to conduct a risk assessment on nutrition and maternal health, focusing on maternal outcomes during pregnancy, childbirth and up to 24 months after delivery.
2. SACN agreed that, where appropriate, other expert committees would be consulted and asked to complete relevant risk assessments. In 2020, a scoping paper was presented to the COT (TOX/2020/45) to define the scope of the work from a toxicological safety perspective and request their input on the selection of candidate chemicals or chemical classes that could be added or removed.
3. In December 2024, the COT requested clarification on the scope of SACN’s nutrition and maternal health project, particularly regarding the stages of the reproductive and developmental cycle assessed. Annex A to TOX/2025/44 was jointly prepared by the FSA and SACN Secretariat in response to this request.
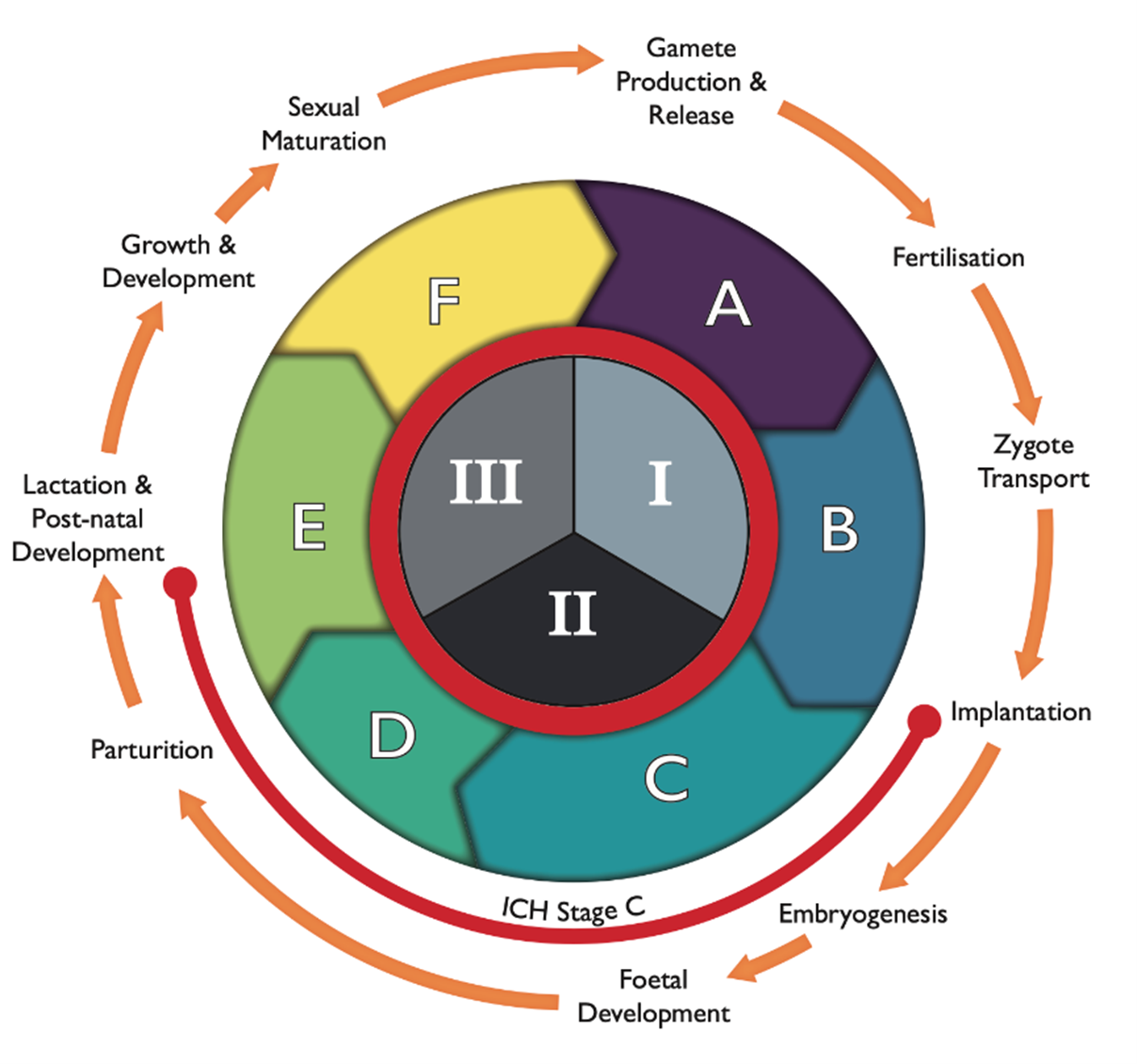
Figure 1: The reproductive and developmental cycle, adapted from IPCS (2001) and Spielmann (2009). The graph also includes the testing strategy by the FDA (1966; ICH, S5(R3), 2021), i.e. segments I (fertility), II (embryotoxicity/teratogenicity) and III (peri-post-natal toxicity), and stages A (pre-mating conception), B (conception to implementation), C (implementation to closure of the hard palate), D (closure of hard palate to end of pregnancy), E (birth to weaning) and F (weaning to sexual maturity). Stage C spans both segment II and III, as indicated by the orange line.
4. Annex A incorporates a diagram representing the stages of the reproductive and developmental cycle (Figure 1), a list of reproductive end points identified by SACN, a proposed list of chemicals to be considered by the COT and the list of completed COT statements on the maternal diet project.
5. The reproductive and developmental cycle schematic (Figure 1) was recommended for inclusion in the Annex by COT Members in December 2024. However, the SACN Secretariat noted that the figure places considerable emphasis on the offspring, whereas the primary focus of the maternal health project should be on the mother. It was proposed that the figure should be amended to clearly highlight the stages relevant to the mother, including preconception, the three trimesters of pregnancy, lactation, and the post-delivery period.
6. If Members are agreeable, it is envisaged that a finalised annex would be attached to future maternal diet papers and would also form part of the overarching paper when this is completed.
Questions for the Committee
The Committee are asked to consider the following questions:
a) Does the Committee have any comments on the reproductive and developmental cycle schematic presented?
b) Does the Committee agree that the reproductive and developmental cycle schematic should be revised to place greater emphasis on maternal health?
c) Does the Committee have any comments on the proposed end points and list of chemicals for consideration?
d) Does the Committee have any other comments on the Annex?
Secretariat
November 2025
Annex A to TOX/2025/44
1. This Annex contains information on the scope of the Scientific Advisory Committee on Nutrition (SACN) maternal diet and nutrition project. It addresses the proposed endpoints as well as the current list of chemical contaminants and nutrients to be assessed by the Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT).
2. SACN sought the advice of the COT on whether excess exposure to a variety of chemicals would pose a risk to maternal health. Chemicals are only to be covered if they are related to a toxicological mechanism rather than inadequate nutrition. As outlined in Figure 1 below, the nutrition and maternal health project will assess the following stages of the reproductive and developmental cycle: preconception (segment I), pregnancy (segment II) and post-delivery (segment III).
3. SACN has considered preconception (segment I) from a biological perspective, as the critical period spanning from days to weeks before embryo development. However, the COT may wish to define this period more precisely for the purpose of the project. The pregnancy stage (segment II) is defined as the time from implantation to parturition. As for post-delivery (segment III), this includes any maternal outcomes relating to the mother 24 months after delivery. It does not include the ‘growth and development’ and ‘sexual maturation’ substages which were considered to be outside the scope of the project. However, there may be some instances where infant outcomes are addressed, but these will be restricted to the neonatal period (i.e. first few days after birth).
Figure 1: The reproductive and developmental cycle, adapted from IPCS (2001) and Spielmann (2009). The graph also includes the testing strategy by the FDA (1966; ICH, S5(R3), 2021), i.e. segments I (fertility), II (embryotoxicity/teratogenicity) and III (peri-post-natal toxicity), and stages A (pre-mating conception), B (conception to implementation), C (implementation to closure of the hard palate), D (closure of hard palate to end of pregnancy), E (birth to weaning) and F (weaning to sexual maturity). Stage C spans both segment II and III, as indicated by the orange line.
Proposed endpoints
4. The following list sets out the reproductive endpoints identified by SACN to be considered by the from a toxicological perspective, with discussion being needed on other outcomes that the COT may regard as relevant.
- Time to conceive,
- Congenital anomalies,
- Miscarriage,
- Stillbirth,
- Preterm delivery,
- Gestational age at delivery,
- Birthweight and size for gestational age,
- Perinatal and neonatal morbidity and mortality,
- Blood pressure and hypertensive disease in pregnancy, including the risk of gestational hypertension and pre-eclampsia.
- Mental health,
- Glycaemic control, including risk of gestational diabetes,
- Anaemia,
- Bone health, including maternal bone mineral density and bone mass.
The following endpoints were considered to be outside the scope of the COT
- Mode of delivery,
- Oral health,
- Weight loss, weight gain and weight retention during pregnancy (gestational weight) and up to 24 months after delivery.
Proposed list of chemicals for consideration
5. The Proposed chemicals to be considered by the COT are discussed below. Chemicals are only to be covered if they are related to a toxicological mechanism rather than inadequate nutrition It should be noted that these chemicals may be subject to change, with some new additions being made where SACN and COT see appropriate.
Vitamins and minerals
- Vitamin A, C, D (including calcidiol), E,
- Selenium, iodine.
Heavy metals
- Lead, arsenic, mercury, cadmium. This was subsequently expanded in include a more general consideration of pica behaviour.
Other food components/herbal supplements
- Tea (Green and black), caffeine, ergot alkaloids, raspberry leaf, echinacea, camomile, peppermint, evening primrose oil, dandelion, resveratrol, phytoestrogens.
Dietary contaminants
- Mycotoxins: citrinin, aflatoxins, fumonisins, ochratoxin A, Zearalenone, deoxynivalenol, nivalenol, patulin, T2 and HT2,
- Oily fish.
Process contaminants
- Acrylamide, heterocyclic amines.
Organic contaminants
- Dioxins and dioxin-like polychlorinated biphenyls PCBs,
- Non-dioxin-like (NDL) PCBs,
- Bisphenol A (BPA),
- Pesticides/legacy pesticides.
Completed papers
6. The below table list the chemicals that have been reviewed by the COT and have published final statements.
Table 1. List of chemicals with completed discussion papers and statements published by the COT before the publication of this Annex A (Scope of the Nutrition and maternal health project) *
|
Chemical |
Statement |
Link |
|
Vitamin D |
Statement on the potential effects of excess vitamin D intake during preconception, pregnancy and lactation |
TOX-2021-45 Second draft statement Vitamin D V05
|
|
Iodine |
Statement on the potential effects that excess iodine intake may have during preconception, pregnancy and lactation |
|
|
Vitamin A |
Statement on the effects of excess Vitamin A on maternal health |
|
|
Cadmium |
Statement on the potential risks from cadmium in the maternal diet |
|
|
Effects on Pica during pregnancy |
Discussion paper on the effects of pica during pregnancy |
TOX-2023-06 Discussion on the effects of pica during pregnancy .pdf |
|
Lead |
Statement on the potential risks from lead in the maternal diet |
Lead in the maternal diet_Statement_FINAL Acc V SO Updated Oct 2024.pdf |
|
Raspberry leaf |
Statement on the potential health effects of raspberry leaf tea in the maternal diet |
|
|
Ginger |
Safety of Ginger Supplement Use in Pregnancy |
|
|
Ergot alkaloids |
Statement on the potential risks from ergot alkaloids in the maternal diet |
To be published shortly. |
|
Mercury |
Statement on the effects of mercury on the maternal health |
To be published shortly. |
|
Citrinin |
Statement on the potential risks from citrinin in the maternal diet |
To be published shortly. |
*papers published before publication of Annex A may have slight deviations in the scope of endpoints assessed.
Table 2. List of chemicals with completed discussion papers and statements published with Annex A (Scope of the Nutrition and maternal health project)*
|
Chemical |
Statement |
Link |
|
Echinacea |
Statement on the potential health effects of Echinacea in the maternal diet |
To be published shortly. |
Secretariat
November 2024
References:
FDA (1966). Guidelines for reproduction studies for safety evaluation of drugs for human use. Washington, Department of Health, Education and Welfare. Referenced in: Raheja KL, Jordan A, Fourcroy JL (1988). Food and drug administration guidelines for reproductive toxicity testing. Reproductive Toxicology, 2(3-4), 291-293. https://doi.org/10.1016/0890-6238(88)90034-2
FDA (2021). S5(R3) Detection of reproductive and developmental toxicity for human pharmaceuticals. Guidance for industry. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Guidance for Industry
IPCS (2001). Principles for evaluating health risks to reproduction associated with exposure to chemicals. Environmental Health Criteria, 225. Principles For Evaluating Health Risks To Reproduction Associated With Exposure To Chemicals (EHC 225, 2001)
Spielmann H (2009). The way forward in reproductive/developmental toxicity testing. Alternatives to Laboratory Animals, 37(6), 641-656. https://doi.org/10.1177/026119290903700609
Spielmann H. The Way Forward in Reproductive/Developmental Toxicity Testing. Alternatives to Laboratory Animals. 2009;37(6):641-656. doi:10.1177/026119290903700609